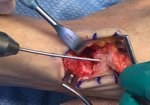
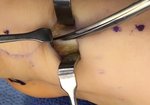
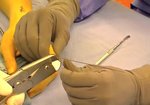
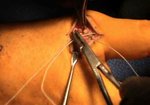
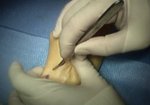
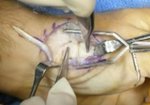
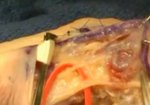
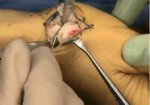
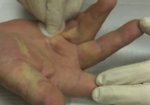
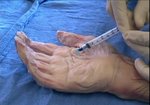
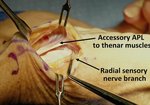
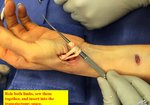
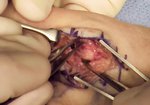
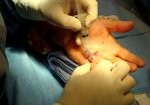
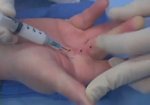
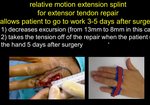
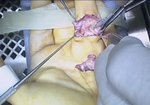
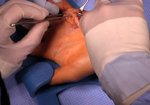
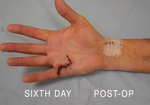
Real Patient Story: Fifth finger 60° MP joint and 45° PIP joint contractures
By
Endo Pharmaceuticals Inc.
FEATURING
Robert Coats
By
Endo Pharmaceuticals Inc.
FEATURING
Robert Coats
INDICATION
XIAFLEX® is indicated for the treatment of adult patients with Dupuytren’s contracture with a palpable cord.
IMPORTANT SAFETY INFORMATION FOR XIAFLEX
- • XIAFLEX is contraindicated in patients with a history of hypersensitivity to XIAFLEX or to collagenase used in any other therapeutic application or application method
- • In the controlled and uncontrolled portions of clinical trials in Dupuytren’s contracture, flexor tendon ruptures occurred after XIAFLEX injection. Injection of XIAFLEX into collagen-containing structures such as tendons or ligaments of the hand may result in damage to those structures and possible permanent injury such as tendon rupture or ligament damage. Therefore, XIAFLEX should be injected only into the collagen cord with a metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contracture, and care should be taken to avoid injecting into tendons, nerves, blood vessels, or other collagen-containing structures of the hand. When injecting a cord affecting a PIP joint of the fifth finger, the needle insertion should not be more than 2 to 3 mm in depth and avoid injecting more than 4 mm distal to the palmar digital crease
- • Other XIAFLEX-associated serious local adverse reactions in the controlled and uncontrolled portions of the clinical studies included pulley rupture, ligament injury, complex regional pain syndrome (CRPS), sensory abnormality of the hand, and skin laceration (tear). In a historically controlled post-marketing trial, the incidence of skin laceration (22%) was higher for subjects treated with two concurrent injections of XIAFLEX compared with subjects treated with up to three single injections in the placebo-controlled premarketing trials (9%). Post-marketing cases of skin laceration requiring skin graft after finger extension procedures and local skin and soft-tissue necrosis, some requiring skin grafting, or other surgical interventions including finger amputation have been reported. Signs or symptoms that may reflect serious injury to the injected finger/hand should be promptly evaluated because surgical intervention may be required
- • Cases of syncope and presyncope have been reported in the post-marketing period in patients treated with XIAFLEX. In most cases in patients with Dupuytren’s contracture, the injection procedure, finger extension procedure, or pain following the procedures were reported as potential triggers for the events, suggesting a vasovagal mechanism. Most, but not all, cases in patients with Dupuytren’s contracture occurred in the immediate treatment period (injection or finger extension procedure) or within 1 to 2 days following the injection or finger extension procedure. If presyncopal symptoms occur, patients should remain recumbent until symptoms resolve. Syncope may be associated with bodily injuries, including concussion, head abrasion, and other accidental injuries
- • In the controlled portions of the clinical trials in Dupuytren’s contracture, a greater proportion of XIAFLEX-treated patients (15%) compared to placebo-treated patients (1%) had mild allergic reactions (pruritus) after up to 3 injections. The incidence of XIAFLEX-associated pruritus increased after more XIAFLEX injections in patients with Dupuytren’s contracture
- • Because XIAFLEX contains foreign proteins, severe allergic reactions to XIAFLEX can occur. Anaphylaxis was reported in a post-marketing clinical trial in one patient who had previous exposure to XIAFLEX for the treatment of Dupuytren’s contracture. Healthcare providers should be prepared to address severe allergic reactions following XIAFLEX injections
- • In the XIAFLEX trials in Dupuytren’s contracture, 70% and 38% of XIAFLEX-treated patients developed an ecchymosis/contusion or an injection site hemorrhage, respectively. Patients with abnormal coagulation (except for patients taking low-dose aspirin, eg, up to 150 mg per day) were excluded from participating in these studies. Therefore, the efficacy and safety of XIAFLEX in patients receiving anticoagulant medications (other than low-dose aspirin, eg, up to 150 mg per day) within 7 days prior to XIAFLEX administration is not known. In addition, it is recommended to avoid use of XIAFLEXin patients with coagulation disorders, including patients receiving concomitant anticoagulants (except for low-dose aspirin)
- • In the XIAFLEX clinical trials for Dupuytren’s contracture, the most common adverse reactions reported in ≥25% of patients treated with XIAFLEX and at an incidence greater than placebo were edema peripheral (eg, swelling of the injected hand), contusion, injection site hemorrhage, injection site reaction, and pain in the injected extremity.
- • Post-marketing experience – Syncope and presyncope have been reported in patients treated with XIAFLEX. Most, but not all, cases occurred in the immediate treatment period or within 1 to 2 days following injection. Bodily injuries associated with the syncopal events have been reported
Please see the full Prescribing Information, including Medication Guide.
XD-06886/May 2023