Video Player is loading.
Current Time 0:00
/
Duration 0:00
Loaded: 0%
0:00
Stream Type LIVE
1x
- 0.5x
- 0.75x
- 1x, selected
- 1.25x
- 1.5x
- 1.75x
- 2x
- Chapters
- descriptions off, selected
- captions settings, opens captions settings dialog
- captions off, selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
10 seconds
Playback speed
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
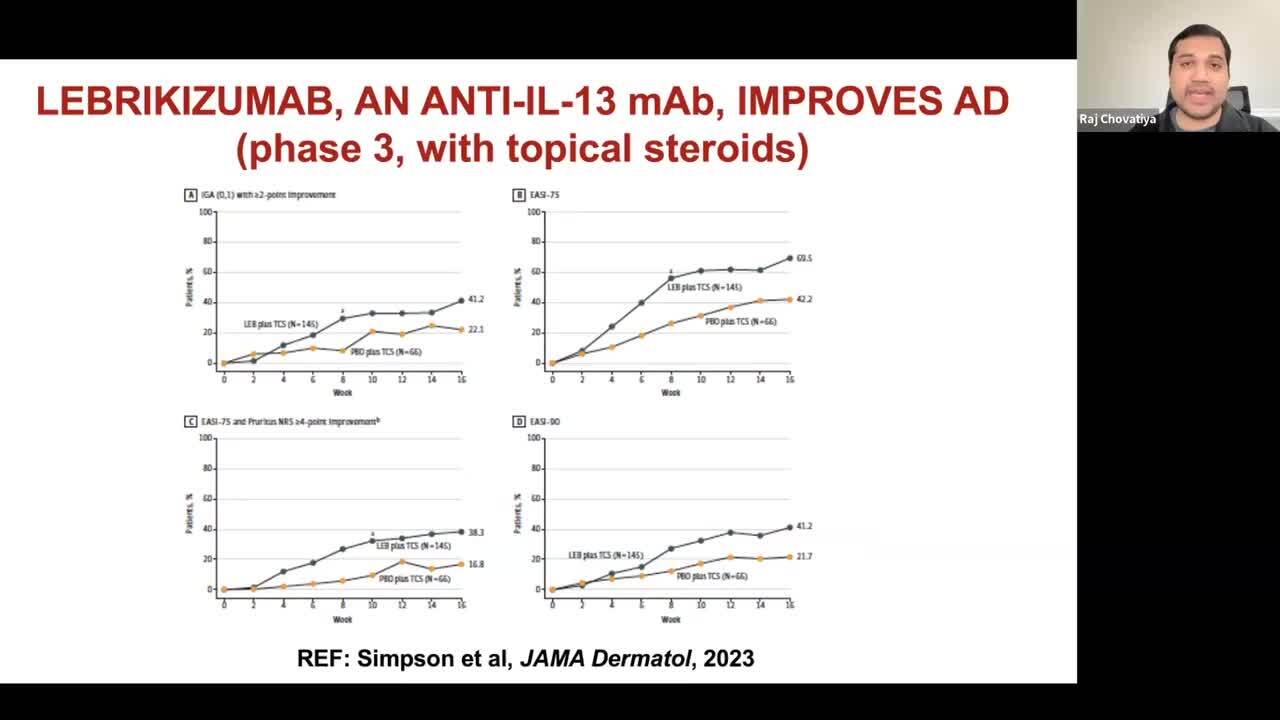
Lebrikizumab Phase 3 Trials: ADvocate1 and ADvocate2 for Atopic Dermatitis
518 views
October 26, 2023
Disclaimer: On September 13, 2024, the U.S. Food and Drug Administration (FDA) approved EBGLYSS™ (lebrikizumab-lbkz), ...
read more ↘ a targeted IL-13 inhibitor, for the treatment of adults and children 12 years of age and older who weigh at least 88 pounds (40 kg) with moderate-to-severe atopic dermatitis (eczema) that is not well controlled despite treatment with topical prescription therapies.
↖ read less
read more ↘ a targeted IL-13 inhibitor, for the treatment of adults and children 12 years of age and older who weigh at least 88 pounds (40 kg) with moderate-to-severe atopic dermatitis (eczema) that is not well controlled despite treatment with topical prescription therapies.
↖ read less
Comments 0
Login to view comments.
Click here to Login



















