Playback speed
10 seconds
- 0.5x
- 0.75x
- 1x, selected
- 1.25x
- 1.5x
- 1.75x
- 2x
- Chapters
- descriptions off, selected
- captions settings, opens captions settings dialog
- captions off, selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
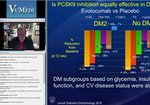
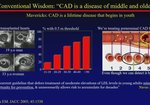
Examining MOA, Safety, and Efficacy Data for a Non-Statin Lipid-Lowering Therapy
INDICATION
LEQVIO (inclisiran) injection is indicated as an adjunct to diet and statin therapy for the treatment of adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), to reduce low-density lipoprotein cholesterol (LDL-C).
IMPORTANT SAFETY INFORMATION
LEQVIO is contraindicated in patients with a prior serious hypersensitivity reaction to inclisiran or any of the excipients in LEQVIO. Serious hypersensitivity reactions have included angioedema. Adverse reactions in clinical trials (≥3% of patients treated with LEQVIO and more frequently than placebo) were injection site reaction, arthralgia, and bronchitis.
Please click here for LEQVIO full Prescribing Information.
Licensed from Alnylam Pharmaceuticals, Inc.
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936-1080 © 2024 Novartis 7/24 FA-11218171