Find latest Neuroendocrine Tumor videos
·
100+ videos

Video Player is loading.
Current Time 0:00
/
Duration 0:00
Loaded: 0%
0:00
Stream Type LIVE
1x
- 0.5x
- 0.75x
- 1x, selected
- 1.25x
- 1.5x
- 1.75x
- 2x
- Chapters
- descriptions off, selected
- captions settings, opens captions settings dialog
- captions off, selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
10 seconds
Playback speed
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
This video is featured in the Insights from 2025 ASCO® GI Annual Meeting
ESMO GI 2024 & ASCO® GI 2025 Insights: Cabozantinib vs. Placebo for R/R Advanced NET - Updated Results From the Ph3 CABINET Trial
79 views
February 18, 2025
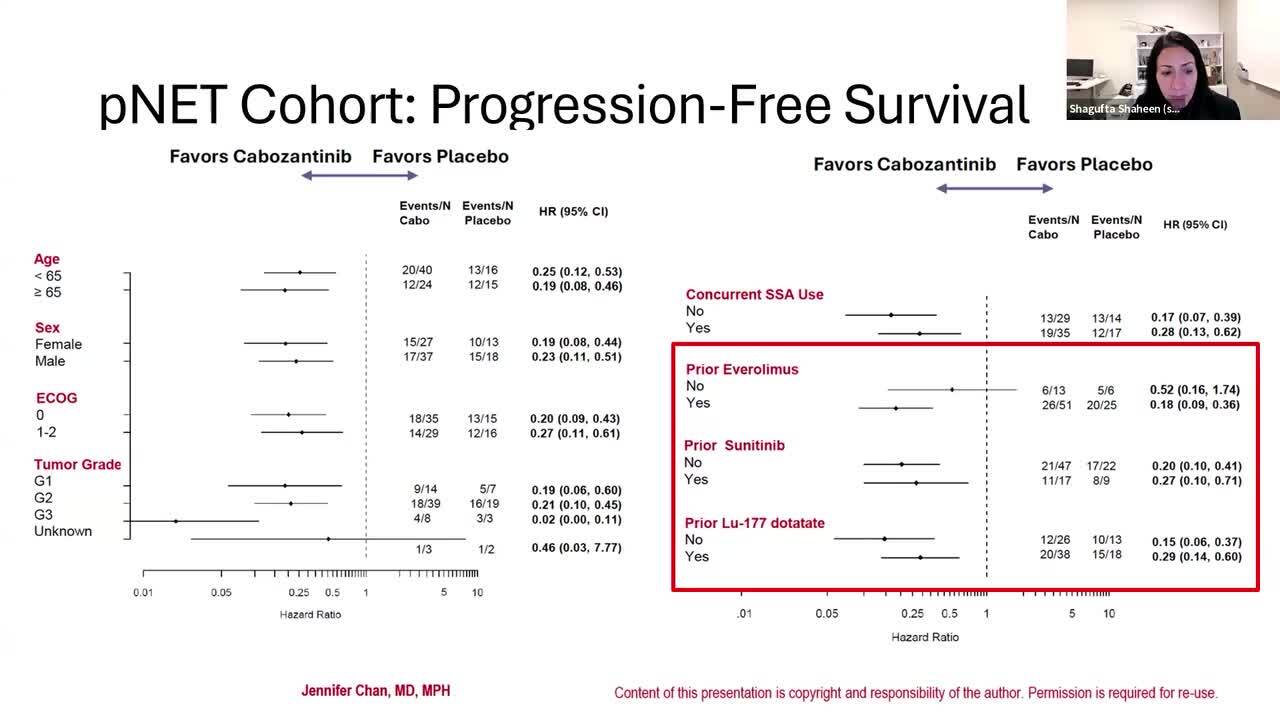
On March 26, 2025, the Food and Drug Administration approved cabozantinib (Cabometyx) for adult and pediatric patients 12 years of age and older with previously treated, unresectable, locally advanced or metastatic, well-differentiated pancreatic neuroendocrine tumors (pNET) and well-differentiated extra-pancreatic neuroendocrine tumors (epNET).
Comments 0
Login to view comments.
Click here to Login


























