Playback speed
10 seconds
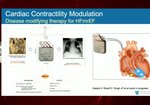
Device Options for Treatment of Heart Failure: Benefits of CCM® Therapy
By
CCM® Therapy
FEATURING
William Abraham
By
CCM® Therapy
FEATURING
William Abraham
612 views
August 28, 2020
In this video, Dr. William Abraham discusses the clinical evidence and benefits of CCM® therapy for patients with NYHA Class III, EF 25 - 45%, that remain symptomatic despite being treated with Guideline Directed Medical Therapy.
- October 26, 2021 - U.S. Food and Drug Administration approved a modification of labeling for the Optimizer® Smart medical device, allowing the removal of “normal sinus rhythm” (NSR) from the indications for use statement as of 10/26/2021. Please note that this presentation was done prior to this FDA update.
Login to view comments.
Click here to Login