Find latest Central Nervous System videos
·
200+ videos

Video Player is loading.
Current Time 0:00
/
Duration 0:00
Loaded: 0%
0:00
Stream Type LIVE
1x
- 0.5x
- 0.75x
- 1x, selected
- 1.25x
- 1.5x
- 1.75x
- 2x
- Chapters
- descriptions off, selected
- captions settings, opens captions settings dialog
- captions off, selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
10 seconds
Playback speed
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
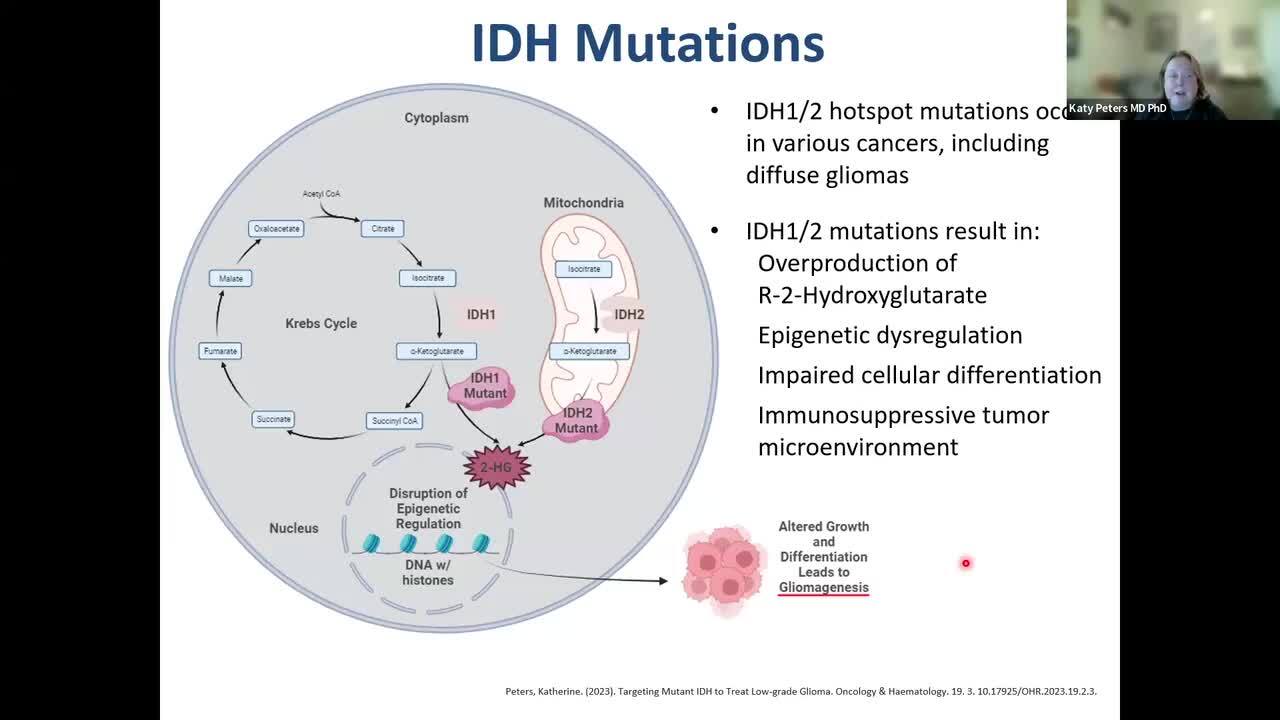
Best Practices for the Provider Approach in Treating Patients With IDH-Mutant Diffuse Glioma
727 views
April 11, 2024
Disclaimer: On August 6, 2024, the FDA approved vorasidenib (Voranigo) for adult and pediatric patients 12 years and older with Grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation, following surgery including biopsy, sub-total resection, or gross total resection.
Comments 0
Login to view comments.
Click here to Login






















