
Shockwave Medical
Shockwave Medical, part of Johnson & Johnson MedTech, is a leader in the development and commercialization of innovative products that are transforming the treatment of cardiovascular disease. Its first-of-its-kind Intravascular Lithotripsy (IVL) technology has transformed the treatment of atherosclerotic cardiovascular disease by using sonic pressure waves to disrupt challenging calcified plaque, resulting in significantly improved patient outcomes. Its Reducer technology, which is under clinical investigation in the United States and is CE Marked in the European Union and the United Kingdom, is designed to provide relief to the millions of patients worldwide suffering from refractory angina by redistributing blood flow within the heart. Learn more at www.shockwavemedical.com.
- 0.5x
- 0.75x
- 1x, selected
- 1.25x
- 1.5x
- 1.75x
- 2x
- Chapters
- descriptions off, selected
- captions settings, opens captions settings dialog
- captions off, selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
10 seconds
Playback speed
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
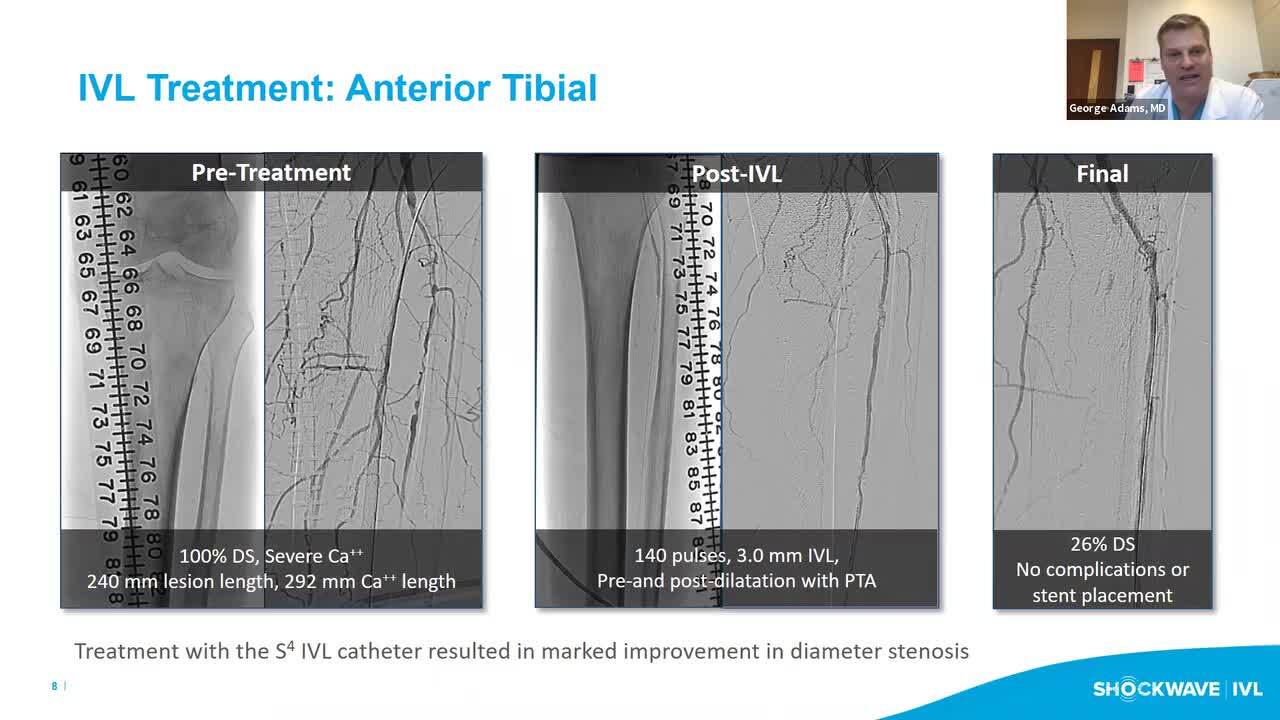
Interim Analysis of the Disrupt PAD III Observational Study: IVL Treatment of Calcified Infrapopliteal Arteries
Important Safety Information
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Indication for Use – The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.
Contraindications – Do not use if unable to pass 0.014 guidewire across the lesion • Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings – Only to be used by physicians who are familiar with interventional vascular procedures • Physicians must be trained prior to use of the device • Use the Generator in accordance with recommended settings as stated in the Operator’s Manual
Precautions – Use only the recommended balloon inflation medium • Appropriate anticoagulant therapy should be administered by the physician • Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology
Adverse Effects – Possible adverse effects consistent with standard angioplasty include: • Access site complications • Allergy to contrast or blood thinners • Arterial bypass surgery • Bleeding complications • Death • Fracture of guidewire or device • Hypertension/Hypotension • Infection/sepsis • Placement of a stent • Renal failure • Shock/pulmonary edema • Target vessel stenosis or occlusion • Vascular complications. Risks unique to the device and its use: • Allergy to catheter material(s) • Device malfunction or failure • Excess heat at target site
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions, and adverse events. www.shockwavemedical.com





























































