
Philips
At Philips, our purpose is to improve people’s health and well-being through meaningful innovation. As a technology company, we innovate for people with one consistent belief: there’s always a way to make life better.
The Philips portfolio of image-guided therapy solutions offers the powerful combination of advanced imaging and specialized treatment options to accurately assess inside the vessel, successfully select the right treatment algorithm and optimize outcomes for your patients. We offer one of the broadest portfolios of interventional solutions in the industry, helping clinicians provide better care for more people, including Philips IVUS and a full portfolio of therapeutic devices that offer specialized treatment options for arterial or venous interventions.
Video Player is loading.
10 seconds
Playback speed
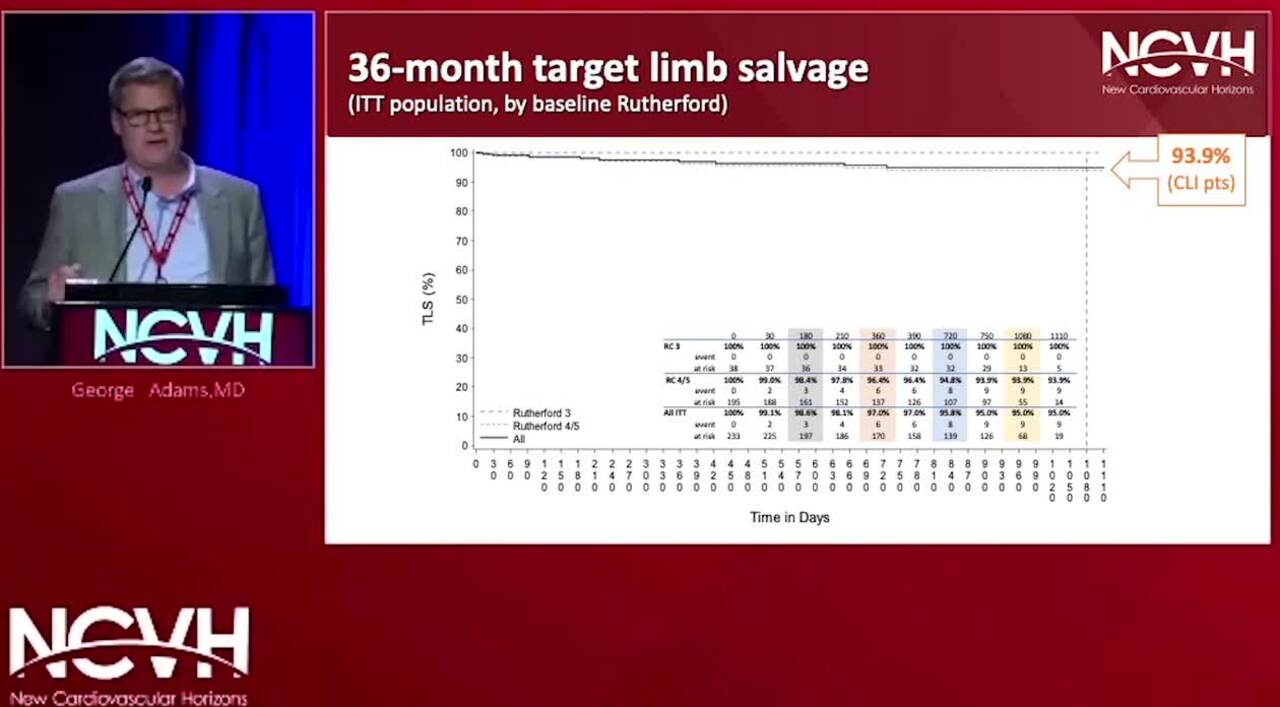
36-month Data on Tack Endovascular System; The Only FDA-approved BTK Scaffold
By
Philips
FEATURING
George Adams
By
Philips
FEATURING
George Adams
Dr. George Adams presents the 36-month data from the TOBA II BTK clinical study for the Tack Endovascular System - the only FDA-approved dissection repair device below-the-knee.
Comments 0
Login to view comments.
Click here to Login


















