
Insights from 2023 ESMO Annual Meeting
Video Player is loading.
10 seconds
Playback speed
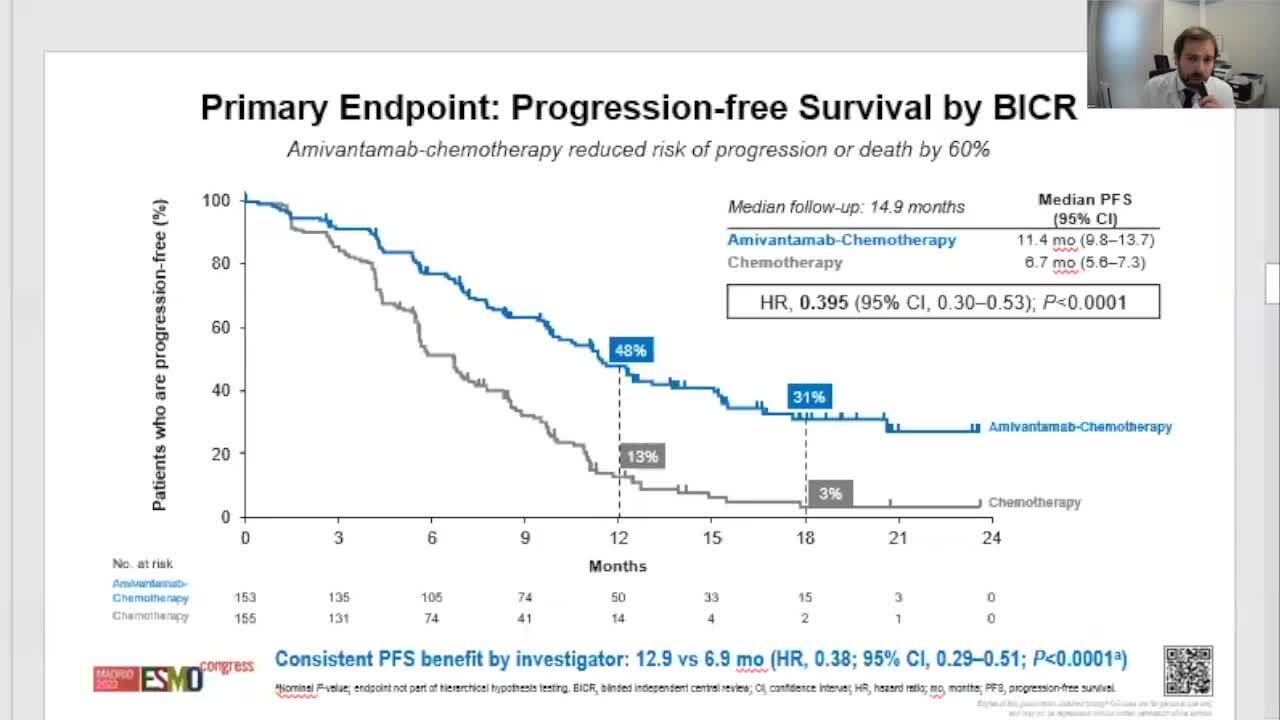
PAPILLON Study - Amivantamab + Chemo vs. Chemo as 1L Treatment in EGFR Exon 20 Insertion-Mutated Advanced NSCLC
By
Insights from 2023 ESMO Annual Meeting
FEATURING
Nicolas Girard
By
Insights from 2023 ESMO Annual Meeting
FEATURING
Nicolas Girard
Disclaimer: On March 1, 2024, The FDA has approved amivantamab-vmjw (RYBREVANT®) plus carboplatin and pemetrexed for the frontline treatment of patients with locally advanced or mNSCLC harboring EGFR exon 20 insertion mutations, as detected by an FDA-approved test.
Comments 0
Login to view comments.
Click here to Login












